一、 研究方向
细胞代谢与表观遗传修饰在肿瘤与衰老中的作用。
肿瘤细胞在细胞代谢方面与正常细胞有着重大区别:正常细胞利用氧化磷酸化高效产生能量,而癌细胞却依赖有氧糖酵解而不是氧化磷酸化来产生能量。许多参与糖酵解的酶在癌细胞中特异性表达而且在癌细胞的生长增殖中起关键作用,已经成为肿瘤治疗的潜在靶位点。针对代谢酶开发的肿瘤治疗方法可以克服普通化疗产生的抗药性、促进化疗引起的癌细胞凋亡。然而,由于大多数代谢酶在肿瘤发生中的作用机制尚不完全清楚,肿瘤代谢治疗方法在应用方面受到了极大的限制。
近来研究表明细胞代谢包括代谢酶和代谢产物可以直接调控表观遗传修饰,比如DNA甲基化、组蛋白乙酰化、组蛋白甲基化等。表观遗传修饰在肿瘤发生和细胞衰老中起重要作用。更重要的是,表观遗传改变在理论上来讲是可逆的,因此有利于开发抗肿瘤和抗衰老的药物。为全面了解代谢酶的功能,本实验室主要以从下述三个方向进行研究:
1、细胞代谢酶以及代谢产物在肿瘤发生中的作用以及表观遗传调控机制。
2、细胞代谢酶以及代谢产物在衰老中的作用以及表观遗传调控机制。
3、抗肿瘤以及抗衰老药物的筛选与化学干预。
二、 团队成员

李珊珊,教授,博士生导师。教育部“青年长江学者”,湖北省“百人计划”特聘教授,湖北省“楚天学者计划”,湖北省杰出青年基金获得者。现为中国老年学和老年医学学会老年病分会衰老基础医学专家委员会常务委员、全国生物化学与分子生物学教学青年委员会委员、湖北省青年科技工作者常务理事、遗传杂志 编委。主要从事糖代谢调控衰老和肿瘤发生的表观遗传机制的研究,近5年以通讯作者在Nature Metabolism、Nature Structural & Molecular Biology、Nature Communications (3篇)、Science Advances、Cell Discovery、Autophagy、Nucleic Acids Research (2篇)、Oncogene等国际知名权威期刊上发表20余篇高水平文章,并获得国家自然科学基金面上项目、青年项目、湖北省创新群体、湖北省杰青项目等多项国家级和省部级科研项目的支持。
Email: shl@hubu.edu.cn
 余希岚,教授,博士生导师。湖北省“百人计划”特聘教授,湖北省杰出青年基金获得者。中国生物化学与分子生物学会基因专业委员会委员,中国老年学和老年医学学会老年病分会衰老基础医学专家委员会委员。主要从基因表达调控角度,深入研究了糖代谢感知通路、糖酵解酶和代谢产物调控细胞衰老和肿瘤发生的表观遗传机制。在国际著名期刊PNAS、Nature Structural & Molecular Biology 、Nature Communications、Science Advances、Cell Discovery、Autophagy、Nucleic Acids Research、等发表SCI论文16篇,主持与参与教科研项目8项。
余希岚,教授,博士生导师。湖北省“百人计划”特聘教授,湖北省杰出青年基金获得者。中国生物化学与分子生物学会基因专业委员会委员,中国老年学和老年医学学会老年病分会衰老基础医学专家委员会委员。主要从基因表达调控角度,深入研究了糖代谢感知通路、糖酵解酶和代谢产物调控细胞衰老和肿瘤发生的表观遗传机制。在国际著名期刊PNAS、Nature Structural & Molecular Biology 、Nature Communications、Science Advances、Cell Discovery、Autophagy、Nucleic Acids Research、等发表SCI论文16篇,主持与参与教科研项目8项。
Email: yuxl(at)hubu.edu.cn
 王姗姗,2020年6月毕业于华中农业大学动物遗传育种与繁殖专业,获农学博士学位。2020年7月-2022年9月在华南农业大学动物科学学院从事博士后研究。2022年11月进入湖北大学生命科学学院任教。目前的研究领域为糖代谢酶和代谢产物通过影响表观遗传修饰调控衰老。在Cell Death & Disease等国际知名学术期刊上发表SCI论文11篇。主持两项省部级科学基金项目。
王姗姗,2020年6月毕业于华中农业大学动物遗传育种与繁殖专业,获农学博士学位。2020年7月-2022年9月在华南农业大学动物科学学院从事博士后研究。2022年11月进入湖北大学生命科学学院任教。目前的研究领域为糖代谢酶和代谢产物通过影响表观遗传修饰调控衰老。在Cell Death & Disease等国际知名学术期刊上发表SCI论文11篇。主持两项省部级科学基金项目。
Email: Wss@hubu.edu.cn

许鑫璘,2021年6月毕业于北京大学生命科学专业,获理学博士学位。2021年7月-2023年6月在北京大学生命科学学院从事博士后研究。2023年6月进入湖北大学生命科学学院任教。目前的研究方向为表观遗传修饰调控衰老的机制研究以及基因的损伤修复与肿瘤治疗的研究。在Nature Structural & Molecular Biology、EMBO reports、elife等国际知名学术期刊上发表SCI论文5篇。参与多项国家级科研项目。
Email: xinlin@hubu.edu.cn

池江洋,2019年12月毕业于华中科技大学临床检验与诊断学专业,获医学博士学位。2020年1月-2023年6月在华中科技大学附属协和医院从事博士后研究。2023年6月进入湖北大学生命科学学院任教。目前的研究方向为糖代谢酶和代谢产物通过表观遗传调控肿瘤。
Email: chijiangyang@163.com

吴银盛,博士后,2023年6月毕业于湖北大学生物化学与分子生物学专业,获理学博士学位。主要的研究方向为糖代谢酶和代谢产物通过影响表观遗传修饰调控肿瘤发生和细胞衰老。在Nature Metabolism、Nature Communications、Nucleic Acids Research等国际知名学术期刊上发表SCI论文8篇。
Email: yinshengwu@foxmail.com

余奇,博士后,2023年6月毕业于湖北大学生物化学与分子生物学专业,获理学博士学位。主要研究方向是糖代谢酶和代谢产物通过影响表观遗传修饰调控基因表达和细胞寿命。在Nature Communications、Science Advances等国际知名学术期刊上发表论文12篇。
Email: youkiey@stu.hubu.edu.cn
三、 代表性研究成果
(一)揭示了糖代谢调控细胞衰老的表观遗传机制
1. 发现丙酮酸激酶复合物通过抑制自噬,调控端粒结构和细胞衰老的表观遗传机制。
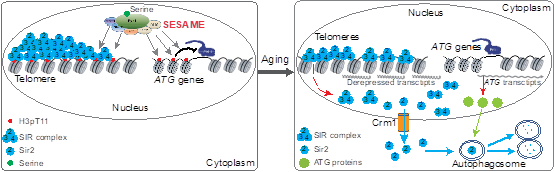
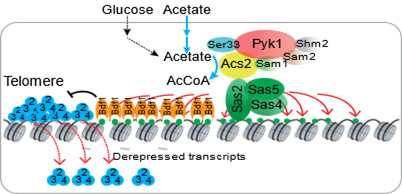
2. 揭示醋酸破坏端粒结构从而促进细胞衰老的表观遗传机制。
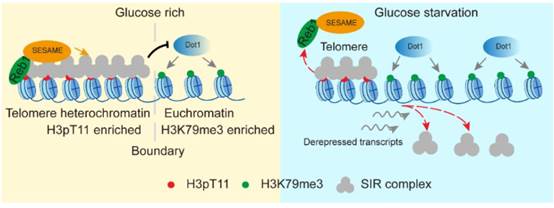
3. 揭示了糖代谢通过组蛋白修饰之间的互作特异性调控端粒结构与细胞衰老的分子机制。
(二)组蛋白调控衰老与肿瘤发生机制
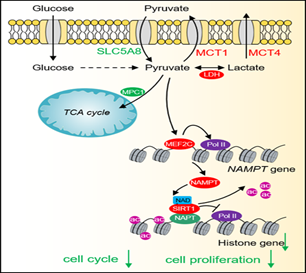
1. 发现丙酮酸作为信号分子抑制组蛋白表达阻止肿瘤生长,揭示了丙酮酸作为抗肿瘤药物的潜力。
2. 发现组蛋白甲基转移酶Set1通过促进组蛋白基因表达延缓细胞衰老。

(三)组蛋白修饰酶感应胞外营养变化分子机制
1. 发现在外界营养发生变化情况下,组蛋白修饰酶SAGA通过自身乙酰化,正协同调控基因转录从而提高细胞抵抗环境压力的能力。
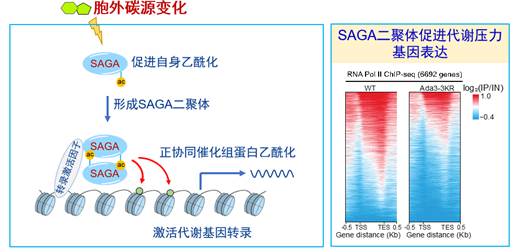
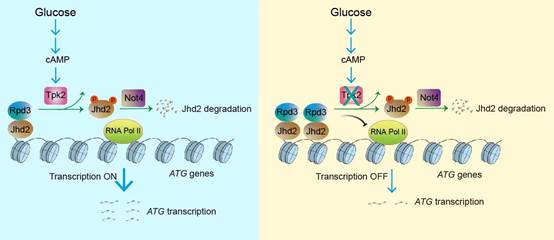
2. 发现营养感知器PKA蛋白激酶家族的催化亚基Tpk2可以抑制组蛋白去甲基转移酶Jhd2的酶活性,促进细胞里的组蛋白H3K4三甲基化, 以适应环境中的营养变化和代谢压力。
四、 科研项目
1. 湖北省自然科学基金创新群体(2021CFA013):血管衰老的表观遗传机制研究2021.10–2024.09。
2. 国家自然科学基金(31970578),丙酮酸通过抑制组蛋白表达调控肿瘤细胞生长的机制研究;2020.1–2023.12。
3. 国家自然科学基金(31872812):SAGA催化的Ada3乙酰化调控基因表达的机制研究;2019.01–2022.12。
4. 湖北省自然科学基金“杰出青年”项目(2019CFA077):丙酮酸激酶PKM2调控血管细胞衰老的表观遗传机制研究;2019.09–2022.09。
5. 广东省基础与应用基础研究基金区域联合基金项目(2020A1515110319),长链非编码RNA NEAT1在肌纤维类型转化中的作用及其分子机制研究,2020.10-2022.10。
6. 中国博士后科学基金面上项目(2021M691075),调控猪肌纤维类型转化的关键m6A修饰lncRNAs筛选及其功能和机制研究,2021.6-2022.9。
7. 国家自然科学基金(31673115):丙酮酸激酶调控端粒异染色质基因沉默的机制研究;2017.1–2020.12。
8. 湖北省自然科学基金“杰青”项目(2017CFA066):二甲双胍抗肿瘤的表观遗传调控的机制研究;2017.1–2019.12。
9. 国家自然科学基金(31600046):丁香假单胞菌海藻糖合成与抗水分胁迫相关性研究;2017.01–2019.12。
10. 生物资源绿色转化湖北省协同创新中心专项启动经费,2015.12-2018.12。
11. 湖北省“青年百人计划”配套经费,2017.07–2020.07。
12. 湖北省自然科学基金(2016CFB103):丙酮酸激酶复合体(SESAME)对端粒异染色质形成以及细胞衰老的影响;2016.01–2017.12。
五、 论文
1. Xinyu Zhang , Qi Yu , Yinsheng Wu , Yuan Zhang , Yi He , Rongsha Wang , Xilan Yu * , Shanshan Li *. Glc7/PP1 dephosphorylates histone H3T11 to regulate autophagy and telomere silencing in response to nutrient availability. Cell Discovery. 9.1(2023).
2. Xin Li, Qianyun Mei, Qin Yu, Min Wang, Fei He, Duncheng Xiao, Hun Liu, Feng Ge, Xilan Yu *, Shanhan Li *. The TORC1 activates Rpd3L complex to deacetylate Ino80 and H2A.Z and repress autophagy. Science Advances. 2023b, 9: eade8312
3. Yinsheng Wu#, Lixu Tang#, Han Huang, Qi Yu, Bicheng Hu, Gang Wang, Feng Ge, Tailang Yin *, Shanshan Li *, Xilan Yu *. Phosphoglycerate dehydrogenase activates PKM2 to phosphorylate histone H3T11 and attenuate cellular senescence. Nature Communications. 2023, 14: 1323
4. Xin Li#, Shanshan Wang#, Xilan Yu *, Shanshan Li *. Transcriptional regulation of autophagy by chromatin remodeling complex and histone variant. Autophagy. 2023a, : 1-3
5. Fei He#, Qi Yu#, Min Wang, Rongsha Wang, Xuanyunjing Gong, Feng Ge, Xilan Yu*, Shanshan Li*. (2022) SESAME-catalyzed H3T11 phosphorylation inhibits Dot1-catalyzed H3K79me3 to regulate autophagy and telomere silencing. Nature Communications, 2022, 13: 7526.
6. Junhua Huang#, Wenjing Dai#, Duncheng Xiao, Qian Xiong, Feng Ge, Xilan Yu*, Shanshan Li*. (2022) Acetylation-dependent dimerization of SAGA complex promotes nucleosome acetylation and gene transcription. Nature Structural & Molecular Biology, 29(3):261-273.
7. Qi Yu#, Xuanyunjing Gong#, Yue Tong, Min Wang, Kai Duan, Xinyu Zhang, Feng Ge, Xilan Yu*, Shanshan Li*. (2022) Phosphorylation of Jhd2 by the Ras-cAMP-PKA(Tpk2) pathway regulates histone modifications and autophagy. Nature Communications, 2022, 13: 5675.
8. Shanshan Wang, Baohua Tan, Liyao Xiao, Xinming Zhao, Jiekang Zeng, Linjun Hong, Jie Yang, Gengyuan Cai, Enqin Zheng, Zhenfang Wu*, Ting Gu*, Comprehensive analysis of long noncoding RNA modified by m(6)A methylation in oxidative and glycolytic skeletal muscles. International Journal of Molecular Science, 2022. 23(9).
9. Shanshan Wang, Baohua Tan, Liyao Xiao, Jiekang Zeng, Xinming Zhao, Linjun Hong, Zicong Li, Gengyuan Cai, Enqin Zheng, Ting Gu*, Zhenfeng Wu*. Long non-coding RNA Gm10561 promotes myogenesis by sponging miR-432. Epigenetics, 2022, 1-17.
10. Jiaxin Qiao, Shanshan Wang, Jian Zhou, Baohua Tan, Zicong Li, Enqin Zheng, Gengyuan Cai, Zhenfang Wu, Linjun Hong*, Ting Gu*, ITGB6 inhibits the proliferation of porcine skeletal muscle satellite cells. Cell Biology International, 2022, 46(1): 96-105.
11. Wanping Chen#, Xilan Yu#, Yinsheng Wu, Qi Yu, Xiaodong Lv, Zitong Zha, Bicheng Hu, Xin Li, Jianguo Chen, Lixin Ma, Jerry L. Workman, Shanshan Li*. The SESAME complex regulates cell senescence through the generation of acetyl-CoA. Nature Metabolism, 2021, 3(7):983-1000.
12. Rui Ma#, Yinsheng Wu#, Shanshan Li*, Xilan Yu*. Interplay between glucose metabolism and chromatin modifications in cancer. Frontiers in Cell and Developmental Biology, 2021, 9:654337.
13. Shihao Zhang#, Xilan Yu#, Yuan Zhang, Xiangyan Xue, Qi Yu, Zitong Zha, Madelaine Gogol, Jerry L. Workman, Shanshan Li*. Metabolic regulation of telomere silencing by SESAME complex-catalyzed H3T11 phosphorylation. Nature Communications, 2021, 12(1):594.
14. Xuanyunjing Gong#, Qi Yu#, Kai Duan, Yue Tong, Xinyu Zhang, Qianyun Mei, Li Lu, Xilan Yu*, Shanshan Li*. Histone acetyltransferase Gcn5 regulates gene expression by promoting the transcription of histone methyltransferase SET1. BBA-Gene Regulatory Mechanisms, 2020, 1863(9):194603.
15. Shanshan Wang, Xuewen Xu, Yan Liu, Jianjun Jin, Feng Zhu, Wei Bai, Yubo Guo, Jiali Zhang, Hao Zuo, Zaiyan Xu, Bo Zuo*, RIP-Seq of EZH2 identifies TCONS-00036665 as a regulator of myogenesis in pigs. Frontiers in Cell and Developmental Biology, 2020, 8: 618617.
16. Rui Ma#, Yinsheng Wu#, Yansheng Zhai, Bicheng Hu, Wei Ma, Wenqiang Yang, Qi Yu, Zhen Chen, Jerry L. Workman, Xilan Yu*, Shanshan Li*. Exogenous pyruvate represses histone gene expression and inhibits cancer cell proliferation via the NAMPT-NAD+-SIRT1 pathway. Nucleic Acids Research, 2019, 47(21): 11132-11150.
17. Qianyun Mei, Chen Xu, Gogol Madelaine, Jie Tang, Wanping Chen, Xilan Yu, Jerry L. Workman*, Shanshan Li*. Set1-catalyzed H3K4 trimethylation antagonizes the HIR/Asf1/Rtt106 repressor complex to promote histone gene expression and chronological life span. Nucleic Acids Research, 2019, 47(7):3434-3449.
18. Shanshan Wang, Hao Zuo, Jianjun Jin, Wei Lv, Zaiyan Xu, Yonghui Fan, Jiali Zhang, Bo Zuo*, Long noncoding RNA Neat1 modulates myogenesis by recruiting Ezh2. Cell Death & Disease, 2019, 10(7): 505.
19. Yinsheng Wu#, Shihao Zhang#, Qi Yu#, Xuanyunjing Gong#, Mingdan Luo, Yuan Zhang, Jerry L. Workman, Xilan Yu*, Shanshan Li*. Glycolysis regulates gene expression by promoting the crosstalk between H3K4me3 and H3K14ac in Saccharomyces cerevisiae. Journal of Genetics and Genomics, 2019, 46(12):561-574.
20. Shanshan Wang, Jianjun Jin, Zaiyan Xu, Bo Zuo*, Functions and regulatory mechanisms of lncRNAs in skeletal myogenesis, muscle disease and meat production. Cells, 2019. 8(9): 1107.
21. Qianruo Wang, Xin Xiu, Wang Ting, Wan Jiawu, Ou Yangtao, Yang Zibing, Yu Qijia, Zhu Liting, Guo Yunli, Wu Yinsheng, Ding Zhen, Zhang Yanni, Pan Zishu, Tang Yuxin, Shanshan Li*, Lingbao Kong*. Japanese encephalitis virus induces apoptosis and encephalitis by activating the PERK pathway. Journal of Virology, 2019, 93:e00887-19.
22. Xilan Yu, Rui Ma, Yinsheng Wu, Yansheng Zhai, Shanshan Li*. Reciprocal regulation of metabolic reprogramming and epigenetic modifications in cancer. Frontiers in Genetics, 2018, 9:394.
23. Fenglin Guo, Xilan Yu, Ahui Xu, Jing Xu, Qianruo Wang, Yunli Guo, Xiaoyu Wu, Yuxin Tang, Zhen Ding, Yanni Zhang, Tian Gong, Zishu Pan, Shanshan Li*, Lingbao Kong*. Japanese encephalitis virus induces apoptosis by inhibiting Foxo signaling pathway. Veterinary Microbiology, 2018, 220: 73-82.
24. Xilan Yu, and Shanshan Li*. Non-metabolic functions of glycolytic enzymes in tumorigenesis. Oncogene, 2017, 36(19):2629-2636.
25. Yi Zheng, Bicheng Hu, Shenggao Xie, Xiaofan Chen, Yuqian Hu, Wanping Chen, Shanshan Li*, Bo Hu*. Dendritic cells infected by Ad-sh-SOCS1 enhance cytokine-inducedkiller (CIK) cell immunotherapeutic efficacy in cervical cancer models. Cytotherapy, 2017, 19: 617-628.
26. Qi Yu, Chong Tong, Mingdan Luo, Xiangyan Xue, Qianyun Mei, Lixin Ma, Xiaolan Yu, Wuxiang Mao, Lingbao Kong, Xilan Yu*, Shanshan Li*. (2017) Regulation of SESAME-mediated H3T11 phosphorylation by glycolytic enzymes and metabolites. PLoS One, 12(4):e0175576.
27. Xilan Yu, Wuxiang Mao, Yansheng Zhai, Chong Tong, Min Liu, Lixin Ma, Xiaolan Yu, Shanshan Li*. Anti-tumor activity of metformin: from metabolic and epigenetic perspectives. Oncotarget, 2017, 8(3):5619-5628.
28. Shanshan Li, Selene K. Swanson, Madelaine Gogol, Laurence Florens, Michael P. Washburn, Jerry L. Workman, Tamaki Suganuma. Serine and SAM responsive complex SESAME regulates histone modification crosstalk by sensing cellular metabolism. Molecular Cell, 2015, 60(3):408-21.
29. Shanshan Li, Lingbao Kong, Xilan Yu, Yi Zheng. Host-virus interactions: From the perspectives of epigenetics. Reviews in Medical Virology, 2014, 24(4): 223-41.
30. Shanshan Li, and Michael A. Shogren-Knaak*. The Gcn5 bromodomain of the SAGA complex facilitates cooperative and cross-tail acetylation of nucleosomes. Journal of Biological Chemistry, 2009, 284(14), 9411-9417.
31. Shanshan Li and Michael A. Shogren-Knaak*. Cross-talk between histone H3 tails produces cooperative nucleosome acetylation. Proc Natl Acad Sci U S A, 2008, 105(47):18243-18248.
32. Shanshan Li*, Lingbao Kong, Xilan Yu. The expanding roles of endoplasmic reticulum stress in virus replication and pathogenesis. Critical Reviews in Microbiology, 2015, 41(2): 150-64.
33. Xilan Yu, Steven P. Lund, Jessica W. Greenwald, Angela H. Records, Russell A. Scott, Dan Nettleton, Steven E. Lindow, Dennis C. Gross, Gwyn A. Beattie*. Transcriptional analysis of the global regulatory networks active in Pseudomonas syringae during leaf colonization. mBio, 2014, 5(5): e01683-14.
34. Xilan Yu, Steven P. Lund, Russell A. Scott, Jessica W. Greenwald, Angela H. Records, Dan Nettleton, Steven E. Lindow, Dennis C. Gross, Gwyn A. Beattie*. Transcriptional responses of Pseudomonas syringae to growth in epiphytic versus apoplastic leaf sites. Proc Natl Acad Sci U S A, 2013, 110(5): E425-E434.
35. Lingbao Kong#,*, Shanshan Li #,*, et al. Oleanolic acid and ursolic acid: novel Hepatitis C virus antivirals that inhibit NS5B activity. Antiviral Research, 2013, 98(1), 44-53.
36. Shanshan Li, Xilan Yu, Gwyn A. Beattie. Glycine betaine catabolism contributes to Pseudomonas syringae tolerance to hyperosmotic stress by relieving betaine-mediated suppression of compatible solute synthesis. Journal of Bacteriology, 2013, 195(10): 2415-23.
37. Lingbao Kong#, Shanshan Li #, Xilan Yu, Xiaonan Fang, Ahui Xu, Mingjie Huang, Xiaoyu Wu, Yunli Guo, Fengli Guo, Jin Xu. Hepatitis C virus and its protein NS4B activate the cancer-related STAT3 pathway via the endoplasmic reticulum overload response. Archives of Virology, 2016, 161(8): 2149-2159.
38. Shanshan Li, Xilan Yu, Yunli Guo, Lingbao Kong. Interaction networks of hepatitis C virus NS4B: Implications for antiviral therapy. Cellular Microbiology, 2012, 14(7): 994-1002.
39. Shanshan Li, Linbai Ye, Xilan Yu, Xu B, Li K, Zhu X, Liu H, Wu X, Kong L. Hepatitis C virus NS4B induces unfolded protein response and endoplasmic reticulum overload response-dependent NF-κB activation. Virology, 2009, 391(2): 257-64.
六、 专利
1. 李珊珊、马瑞等。一种将白藜芦醇和丙酮酸组合用于抗肿瘤药物。专利申请号: 201711156206.3。
2. 李珊珊。一种用于检测组蛋白豆蔻酰化修饰的重组蛋白, 2019-11-8, 中国, ZL201710147487.X.
3. 李珊珊。一种用于检测组蛋白位点乙酰化的重组蛋白及, 2019-12-20, 中国, ZL201710581878.2.
4. 李珊珊。一种用于检测组蛋白三甲基化修饰的重组蛋白, 2019-12-20, 中国, ZL201710582691.4.





